Patient-led research in rare diseases: How can we make this a reality?

Time and again we hear how those with lived experience of a rare disease are the real experts in their own condition. After all, they live daily with the reality of managing, coping and often struggling with its impact. They know only too well what improves their experiences and what makes things worse, so it could be reasonably argued that they are best placed to inform developments in care and treatment designed to support them. Advocacy groups like EURORDIS have been calling for people with lived experience to be at the heart of research and developments aimed at improving their experiences for quite some time.
But are these calls being heard? Are the research questions driving forward our knowledge and understanding of rare diseases coming from the community themselves, or are they primarily driven by those in the ecosystems surrounding them? Is there a way to make research in rare disease truly patient centric and not simply about achieving commercial goals?
Throughout this article, we will explore these questions and consider some of the solutions that already exist to support with patient-led research, while pondering if we are really doing enough!
For many people living with rare diseases, their families and the advocacy groups representing them, research may seem like an unknown entity. On hearing that their child has been diagnosed with a rare, life limiting condition, where little is known of the condition and no current treatments exist, many families become unintentional researchers. They begin their journey into the unknown without fully understanding the magnitude of it all; researching the condition, learning everything they can to be their own advocate; identifying and networking with specialists who understand their condition; seeking out possible treatments and learning about the drug development process; finding other families and individuals with the same condition and forming networks to learn and grow together; embarking on fundraising efforts to support what little research exists; attending scientific conferences and establishing support groups. All of this and much more, whilst often managing full-time jobs, a condition with complex needs, multiple hospital appointments, in a background of little to no medical knowledge (for the vast majority). It is unsurprising that they become the experts in their condition and their voice plays such a critical role in research.
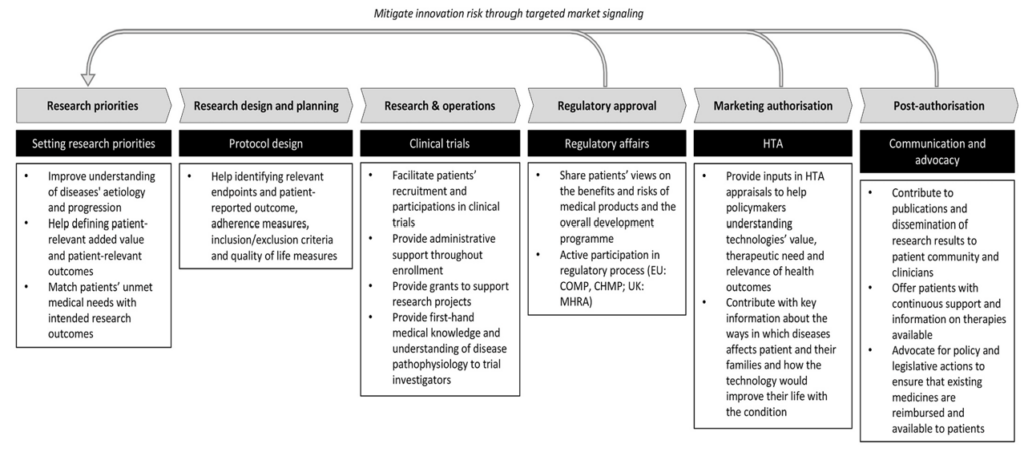
taken from Gentilini & Miraldo (2023)
However, although research suggests that more active patient groups supporting some of the more prevalent rare conditions can play a role in driving forward research into treatments2 and there is increased understanding of the role the patient voice plays in delivering improved outcomes across all stages of the drug discovery process in rare diseases, it seems that researchers are still not maximising this valuable resource. A paper published in the Orphanet Journal of Rare Diseases this month (Feb 2025) suggests that the involvement of patient voice in clinical trials within the rare disease space remains “suboptimal” and despite recognition of the important role it plays across all stages of the drug development process, it is still not happening, which, can negatively impact on outcomes3.
So, how can the voice of lived experience be represented more effectively in research? And what about ensuring that voice is represented beyond participation in clinical trials.
The Patient Led Research Hub was established in 2015 with these very questions in mind. It understood the mismatch between the research questions presented by academic or commercial organisations with those important to the people living with a condition and in 2022 it began to solely focus on research into rare diseases4. This has surely provided an invaluable opportunity for people living in the UK with a rare disease to drive forward research questions important to them and their community, with clear guidance and support on how to progress this idea.
However, one of the key components to this process is the involvement of an active patient organisation. This perhaps limits those with an ultra-rare condition, who do not know anyone else with their condition and who have no active patient support group. What if their research question is about how to find others with the same condition so that they can understand more about the condition, effectively starting a registry? Without a patient group they would be unable to access the PLRH services. Thankfully these sorts of challenges have been considered by the team at CamRare, and the PLRH who listened to the voice of the rare disease community asking for more opportunities for patient -centred research. The Rare Disease Research Network was launched in November 2024 and is “a patient driven research network for all” aimed at “turning research on its head”5.
This network is a platform, facilitating discussions and networking across all sectors of the rare disease ecosystem. It provides a space where individuals can sign up and share their knowledge, experience and expertise or where research teams can be drawn together from this same pool. For individuals with lived experience or representing someone with lived experience, it provides an opportunity to share a research idea, access to knowledge and expertise in the form of mentoring that could help them to shape a fledgling idea into a full-blown patient-led research project and access resources and support that have been created by their peers. The platform has been designed to help those new to research to progress through the various steps of their research journey with a ready source of peer support, helping them to be research ready and take their ideas forward. It is essentially creating a space for the questions and needs of the rare disease community to deliver research that creates outcomes that matter to the community.
If this network is to be a success, it requires support from across our varied rare disease ecosystem. This means people from academia, pharma and biotech, entrepreneurs, health and social care and funding bodies need to sign up and get involved. This network can’t and won’t work if only patients and patient organisations sign up. But the reality is, if we all get involved and share our expertise through the network, we can drive patient-led research outcomes.
Take the example previously suggested of the individual who wants to find more people with their condition, we can draw a very simplified conclusion of the potential the network could have.
Having the ability to connect with people who could mentor them and support them to establish a patient organisation and create a registry of people living with the condition, has benefits for the patient community but in addition it drives patient insights that have the potential to deliver far greater impact. It could inform the natural history of the condition, invaluable in generating greater understanding about the progression of the condition. It provides a greater understanding of the prevalence and incidence of the condition and potential variations in presentation. These factors in turn have the potential to then make that condition much more attractive to commercial organisations and provide the basic building blocks that can start to inform drug development. Clearly this is a very simplistic and crude explanation of what is a much more complex process, however it does highlight that by supporting those with lived experience to answer the questions that matter to them, we can then inform the wider rare disease ecosystem with research that is driven by their needs.
If we want to ensure that the patient voice in rare disease research does not continue to be “suboptimal”, we all need to play our part. Go online and find out more about why you should join the RDRN here and make sure you are part of “turning research on its head!”.
References
- Mavris, M and Le Cam, Y. (2012). Involvement of Patient Orghanisations in Research and Development of Orphan Drugs for Rare Diseases in Europe. Molecular Syndromology. 3: 237-243. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC3531929/pdf/msy-0003-0237.pdf [Accessed 14 February 2025]
- Arianna Gentilini and Mariso Miraldo. (2023). The role of patient organisations in research and development: Evidence from rare diseases. Social Science & Medicine. Available at: The role of patient organisations in research and development: Evidence from rare diseases – ScienceDirect [Accessed 11 February 2025]
- Pijaun, J and Palau, F. (2025). Patient involvement in clinical trials: a paradigm shift in research. Orphanet Journal of Rare Diseases. 20 (63). Available at: Patient involvement in clinical trials: a paradigm shift in research | Orphanet Journal of Rare Diseases | Full Text [Accessed 12th February 2025]
- Patient Led Research Hub. (n.d). About Us. Available at: https://plrh.org/about-us/ [Accessed 14 February 2025]
- Rare Disease Research Network. (n.d). Home. Available at: https://rd-rn.org/ [Accessed 14 February 2025]
About Michelle
Michelle Conway trained as a nurse, before moving from the NHS to the pharmaceutical industry, where she took up a role as a nurse specialist. Michelle discovered a passion for rare disease when working with a biotech organisation to launch a product for an ultra-rare disease. She then supported the company with the launch of several ultra-orphan medicines.
With experience across multiple roles in the biotech and pharmaceutical industries, Michelle has expertise in market access and policy work. Michelle supports projects requiring expertise and knowledge about the challenges of access and policy for medicines for rare cancers and orphan medicine launch-planning.


